Answer:
The the specific volume and weight are 79.16m³/k mol and 49 N.
Step-by-step explanation:
Given that,
Diameter = 3 m
Mass of N₂ = 5 kg
We need to calculate the volume of balloon
Using formula of volume
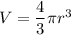

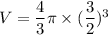
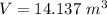
We need to calculate the specific volume in the balloon
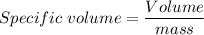
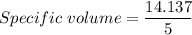
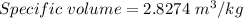
Now, Molar mass of N₂ gas is 0.028 kg/mol
Now, Specific volume of N₂ gas in the balloon is
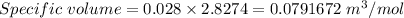
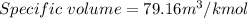
(b). We need to calculate the weight of the gas in the balloon
Weight of the balloon is dependent on the mass of the gas.
The weight of the gas is given by
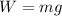
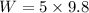
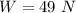
Hence, The the specific volume and weight are 79.16m³/k mol and 49 N.