Answer:
The value of
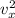 is
is
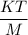 .
.
Step-by-step explanation:
A gas is equilibrium at T kelvin.
Mass = M
We know that,
The average square of the velocity in the x,y and z direction are equal.
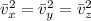
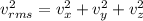
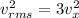
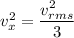
Equation of ideal gas
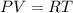
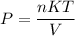
Here, R = nK
We know that,
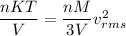
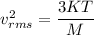 ....(I)
....(I)
Put the value of
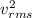 in the equation (I)
in the equation (I)
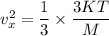
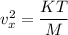
Hence, The value of
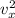 is
is
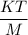 .
.