Answer:
The internal energy is 73.20 J.
Step-by-step explanation:
Given that,
Weight of helium = 86 mg
Temperature = 0°C
We need to calculate the internal energy
Using formula of internal energy
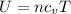
Where,
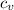 = specific heat at constant volume
= specific heat at constant volume
He is mono atomic.
So, The value of
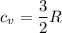
now, 1 mole of Helium = 4 g helium
n =number of mole of the 86 mg of helium
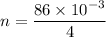
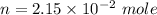
T = 0°C=273 K
Put the value into the formula
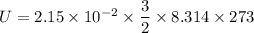
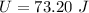
Hence, The internal energy is 73.20 J.