Answer:
Force,
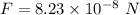
Step-by-step explanation:
It is given that,
Each ion in Na⁺ and Cl⁻ has a charge of,
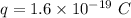
Distance between two ions,
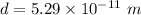
We need to find the electrostatic force. It is given by :
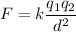
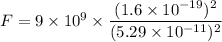
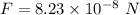
So, the magnitude of electrostatic force between them is
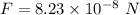 . Hence, this is the required solution.
. Hence, this is the required solution.