Answer:
Approximately
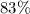 .
.
Step-by-step explanation:
How many moles of
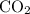 gas are released?
gas are released?
The volume of each mole of of an ideal gas is approximately
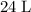 under room temperature and pressure (r.t.p,
under room temperature and pressure (r.t.p,
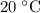 ,
,
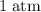 .) That's the same as
.) That's the same as
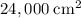 .
.
Assume that
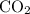 acts like an ideal gas.
acts like an ideal gas.
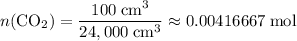 .
.
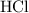 is in excess. How many moles of
is in excess. How many moles of
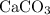 formula units will produce that
formula units will produce that
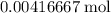 of
of
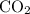 ?
?
Consider the ratio between the coefficient of
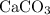 and that of
and that of
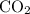 .
.
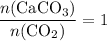 .
.
In other words,
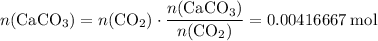 .
.
What's the mass of that many
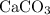 ?
?
Relative atomic mass data from a modern periodic table:
- Ca: 40.078;
- C: 12.011;
- O: 15.999.
Formula mass of
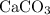 :
:
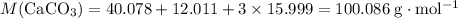 .
.
Mass of that
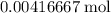 of
of
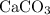 :
:
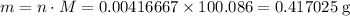 .
.
Percentage mass of
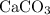 in this sample of chalk:
in this sample of chalk:
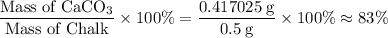 .
.