Hello!
Determine the molarity of a solution formed by dissolving 97.7 g LiBr in enough water to yield 750.0 ml of solution.
We have the following data:
M (Molarity) =? (in mol / L)
m1 (mass of the solute) = 97.7 g
V (solution volume) = 750 ml → V (solution volume) = 0.75 L
MM (molar mass of LiBr)
Li = 6.941 u
Br = 79.904 u
---------------------------
MM (molar mass of LiBr) = 6.941 + 79.904
MM (molar mass of LiBr) = 86.845 g/mol
Now, let's apply the data to the formula of Molarity, let's see:
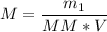
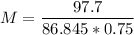
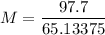
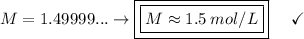
________________________
________________________
*** Another way to solve is to find the number of moles (n1) and soon after finding the molarity (M), let's see:
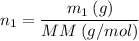

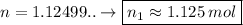
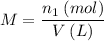
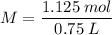
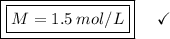
_____________________