Answer:
The pressure of the air is 499.91 kPa.
Step-by-step explanation:
Given that,
Initial pressure = 430 kPa
Temperature = 13.0+273=286 K
Final temperature = 59.5+273=332.5 K
We need to calculate the final pressure
Using relation of pressure and temperature
At constant volume,
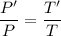
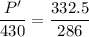
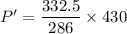
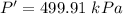
Hence,The pressure of the air is 499.91 kPa.