Answer:
The mass of neon is 13.534 g.
Step-by-step explanation:
Given that,
Volume = 3.8 L
Pressure = 6.8 atm
Temperature = 470 K
Atomic mass of neon =20.2 g/mol
Gas constant R = 8.314 = 0.082057 L atm/mol K
We need to calculate the mass of neon
Using equation of gas

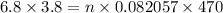
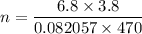
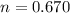
We know that,
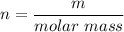
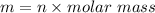
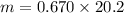
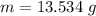
Hence, The mass of neon is 13.534 g.