Answer:
so specific heat capacity of unknown metal is 656.8 J/degree C kg
Step-by-step explanation:
Here by energy balance we can say that energy given by metal alloy at 100 degree = heat absorbed by water at 18.7 degree C
now we have
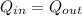
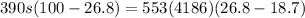
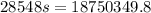
now we have
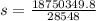
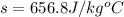
so specific heat capacity of unknown metal is 656.8 J/degree C kg