Answer:
Part a)
h = 0.86 cm
Part b)
Level will increase
Step-by-step explanation:
Part a)
Mass of the ice cube is 0.200 kg
Now from the buoyancy force formula we know that weight of the ice is counter balanced by buoyancy force on the ice
So here we will have
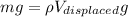

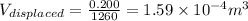
now as we know that ice will melt into water
so here volume of water that will convert due to melting of ice is given as
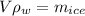
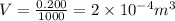
So here extra volume that rise in the level will be given as
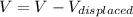
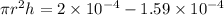
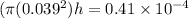
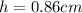
Part b)
Since volume of water that formed here is more than the volume that is displaced by the ice so we can say that level of liquid in the cylinder will increase due to melting of ice