Answer:
542.06 K
Step-by-step explanation:
v = rms speed of oxygen molecule = 650 m/s
M = molecular mass of the oxygen molecule = 32 g = 0.032 kg
R = universal gas constant = 8.314 J/(mol K)
T = temperature of the gas
Rms speed of oxygen molecule is given as
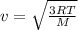
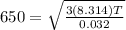
T = 542.06 K