Answer:
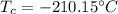
Step-by-step explanation:
In this question we need to convert the temperature in kelvin to degree Celsius. The conversion from kelvin scale to Celsius scale is as follows :
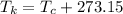
Here,
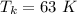
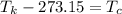
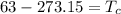
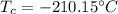
Here, negative sign shows that the heat is released. So, the temperature at 63 K is equivalent to 210.15 °C. Hence, this is the required solution.