Answer:
1.90 x 10² cal
1.90 x 10² cal
0.133 cal/(g °C)
Step-by-step explanation:
For water :
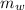 = mass of water = 112 g
= mass of water = 112 g
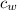 = specific heat of water = 1 cal/(g °C)
= specific heat of water = 1 cal/(g °C)
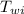 = initial temperature of water = 90.0 °C
= initial temperature of water = 90.0 °C
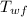 = final temperature of water = 88.3 °C
= final temperature of water = 88.3 °C
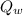 = Heat lost by water
= Heat lost by water
Heat lost by water is given as
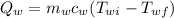
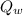 = (112) (1) (90.0 - 88.3)
= (112) (1) (90.0 - 88.3)
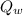 = 1.90 x 10² cal
= 1.90 x 10² cal
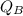 = Heat gained by the block
= Heat gained by the block
As per conservation of energy
Heat gained by the block = Heat lost by water
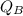 =
=
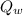
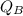 = 1.90 x 10² cal
= 1.90 x 10² cal
For Block :
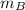 = mass of block = 21.0 g
= mass of block = 21.0 g
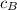 = specific heat of block
= specific heat of block
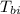 = initial temperature of block = 20.0 °C
= initial temperature of block = 20.0 °C
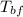 = final temperature of block = 88.3 °C
= final temperature of block = 88.3 °C
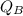 = Heat gained by Block = 1.90 x 10² cal
= Heat gained by Block = 1.90 x 10² cal
Heat gained by water is given as
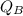 = m_{B}c_{B}(T_{bf} - T_{bi})[/tex]
= m_{B}c_{B}(T_{bf} - T_{bi})[/tex]
1.90 x 10² = (21.0) (88.3 - 20.0) c_{B}
c_{B} = 0.133 cal/(g °C)