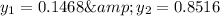Answer:
Mole fraction of alcohols in liquid phase
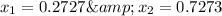 .
.
Mole fraction of alcohols in vapor phase
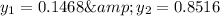 .
.
Step-by-step explanation:
The total vapor pressure of the solution = p =38.6 Torr
Partial vapor pressure of the n-propyl alcohol =
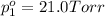
Partial vapor pressure of the isopropyl alcohol =
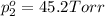
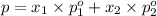 (Raoult's Law)
(Raoult's Law)
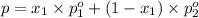
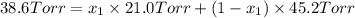
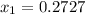
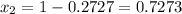
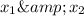 is mole fraction in liquid phase.
is mole fraction in liquid phase.
Mole fraction of components in vapor phase
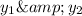
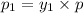 (Dalton's law of partial pressure)
(Dalton's law of partial pressure)
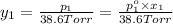
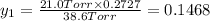
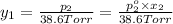
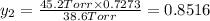
Mole fraction of alcohols in vapor phase