Answer : The mass of ammonia present in the flask in three significant figures are, 5.28 grams.
Solution :
Using ideal gas equation,
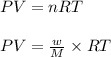
where,
n = number of moles of gas
w = mass of ammonia gas = ?
P = pressure of the ammonia gas = 2.55 atm
T = temperature of the ammonia gas =
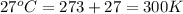
M = molar mass of ammonia gas = 17 g/mole
R = gas constant = 0.0821 L.atm/mole.K
V = volume of ammonia gas = 3.00 L
Now put all the given values in the above equation, we get the mass of ammonia gas.
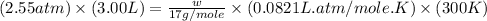
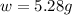
Therefore, the mass of ammonia present in the flask in three significant figures are, 5.28 grams.