Answer: 0.11 M
Step-by-step explanation:
The equation for the reaction is given as:
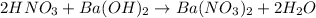
According to the neutralization law,
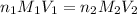
where,
 = molarity of
= molarity of
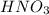 solution = ?
solution = ?
 = volume of
= volume of
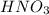 solution = 0.105 L= 105 ml
solution = 0.105 L= 105 ml
 = molarity of
= molarity of
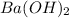 solution = 0.2 M
solution = 0.2 M
 = volume of
= volume of
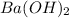 solution = 29.1 ml
solution = 29.1 ml
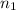 = valency of
= valency of
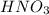 = 1
= 1
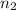 = valency of
= valency of
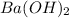 = 2
= 2
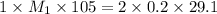
![M_1=0.11M[/tex<strong>] </strong></p><p><strong>Therefore, the concentration of [tex]HNO_3](https://img.qammunity.org/2020/formulas/chemistry/college/12z2wu3c9nga4lkm4glzrvl6yzlh4wr2dd.png) will be 0.11 M.
will be 0.11 M.