Answer:
35.5 amu is the average atomic mass of chlorine
Step-by-step explanation:
Fractional abundance of chlorine‑35 = 75.5%
Fractional abundance of chlorine‑37 = 24.5%
Average atomic mass is equal to summation of products of all isotopes masses into their fractional abundance.
Average atomic mass = Σ(Mass of an isotope × fractional abundance)
Average atomic of chlorine :
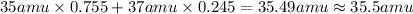
35.5 amu is the average atomic mass of chlorine