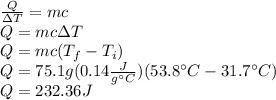Answer:
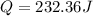
Step-by-step explanation:
The heat capacity (C) of a physical system depends on the amount of substance of that system. For a system formed by a single homogeneous substance, it is defined as:
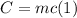
Here m is the mass of the system and c is the specific heat capacity.
The heat capacity is defined as the ratio between the heat absorbed by the system and the resulting temperature change:
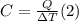
We equal (1) and (2) and solve for Q: