Answer: a mole
Avogadro's number
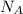 is determined by the number of particles (or atoms) in a mole:
is determined by the number of particles (or atoms) in a mole:
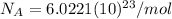
It should be noted that the mole is one of the seven fundamental units of the International System of Units and defines the amount of substance.
Therefore:
Avogadro’s number was calculated by determining the number of atoms in a mole.