Answer:
E. 5
Step-by-step explanation:
N₀ = initial total number of radioactive elements number
N = Number of atoms of radioactive element after "n" half lives = N₀ /32
n = number of half lives
Number of atoms of radioactive element after "n" half lives is given as
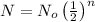
inserting the values
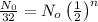
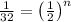
n = 5