Answer:
Part a)
decrease in internal energy is 2.23 k Cal
Part b)
Efficiency will be 4.9 %
Step-by-step explanation:
Part A)
As per first law of thermodynamics we know that
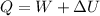
here we have
Q = -8854 J
W = 463 J
now we have
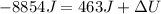
so we have
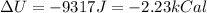
So decrease in internal energy is 2.23 k Cal
Part B)
Efficiency of the woman is given as
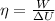
here we have
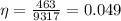
So efficiency will be 4.9 %