Answer:
The temperature of hot reservoir is 669.24 K.
Step-by-step explanation:
It is given that,
Efficiency of gasoline engine,
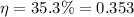
Temperature of cold reservoir,
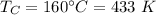
We need to find the temperature of hot reservoir. The efficiency of Carnot engine is given by :
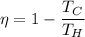
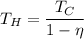
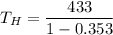
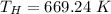
So, the temperature of hot reservoir is 669.24 K. Hence, this is the required solution.