Answer:
0.3097 moles of an nonionizing solute would need to be added.
Step-by-step explanation:
Molal elevation constant =
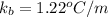
Normal boiling point of ethanol =
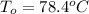
Boiling of solution =
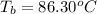
Moles of nonionizing solute = n
Mass of ethanol (solvent) = 47.84 g
Elevation boiling point:
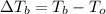
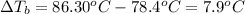
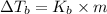
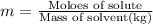
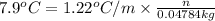
n = 0.3097 mol
0.3097 moles of an nonionizing solute would need to be added.