Answer:
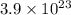 iron atoms
iron atoms
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance weighs equal to the molecular mass and contains avogadro's number
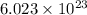 of particles.
of particles.
![1 molecule of [tex]Fe_2O_3](https://img.qammunity.org/2020/formulas/chemistry/college/6w53arxah6w9lehmoensla4tjimpzq7l2p.png) contains= 2 atoms of iron
contains= 2 atoms of iron
![1 mole of [tex]Fe_2O_3](https://img.qammunity.org/2020/formulas/chemistry/college/lo194vnw25n3nwubp0xhqoxufayuffb2kl.png) contains=
contains=
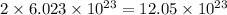 atoms of iron
atoms of iron
thus 0.32 moles of
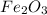 contains=
contains=
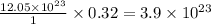 atoms of iron
atoms of iron
Thus the sample would have
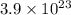 iron atoms.
iron atoms.