Answer : The pH of 0.001 M
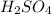 is, 2.69 and the pH after mixing the solution is, 3.30.
is, 2.69 and the pH after mixing the solution is, 3.30.
Explanation :
First we have to calculate the concentration of hydrogen ion.
The balanced dissociation reaction will be,
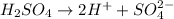
The concentration of
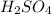 = x = 0.001 M
= x = 0.001 M
The concentration of
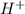 ion = 2x = 2 × 0.001 M = 0.002 M
ion = 2x = 2 × 0.001 M = 0.002 M
The concentration of
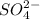 = x = 0.001 M
= x = 0.001 M
Now we have to calculate the pH of 0.001 M
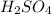 .
.
![pH=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ipfjz05f4cfbguiwup37xvxa7furlbuapf.png)
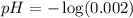
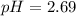
Now we have to calculate the molarity after mixing the solution.
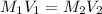
where,
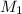 = molarity of
= molarity of
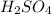 solution = 0.001 M
solution = 0.001 M
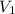 = volume of
= volume of
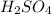 solution = 250 ml
solution = 250 ml
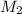 = molarity of after mixing = ?
= molarity of after mixing = ?
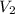 = volume of after mixing = 250 + 750 = 1000 ml
= volume of after mixing = 250 + 750 = 1000 ml
Now put all the given values in the above formula, we get the molarity after mixing the solution.
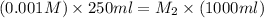
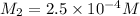
The concentration of
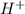 ion =
ion =
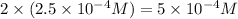
Now we have to calculate the pH after mixing the solution.
![pH=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ipfjz05f4cfbguiwup37xvxa7furlbuapf.png)
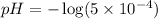
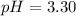
Therefore, the pH of 0.001 M
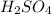 is, 2.69 and the pH after mixing the solution is, 3.30.
is, 2.69 and the pH after mixing the solution is, 3.30.