Answer:
146,575 grams of ethylene glycol would need to be dissolved in 15 kg of pure water
The boiling point of the solution is 181.95°C.
Step-by-step explanation:
Mass of ethylene glycol = x
Molar mass of ethylene glycol = 2 × 12 g/mol + 2 × 32 g/mol+ 4 × 1= 62g/mol
Moles of ethylene glycol =
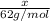
Mass of solvent that is water = 15 kg
The molal freezing point depression constant for water
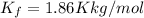
Molality of the solution:
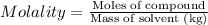
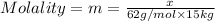
Depression in freezing point of water =
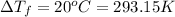
((T)°C =T+ 273.15 K)
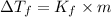
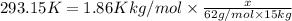
x = 146,575 g
Boiling point of this solution =
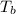
The molal boiling point elevation constant for water
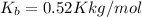
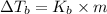
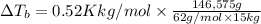
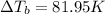
Normal boiling point of water is = T = 373.15 K
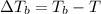
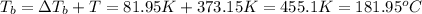
The boiling point of the solution is 181.95°C.