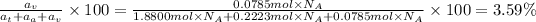Step-by-step explanation:
Suppose in 100 g of alloy contains 90% titanium 6% aluminum and 4% vanadium.
Mass of titanium = 90 g
Moles of titanium =
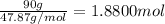
Total number of atoms of titanium ,
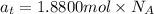
Mass of aluminum = 6 g
Moles of aluminium =
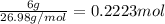
Total number of atoms of aluminium,
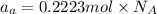
Mass of vanadium = 4 g
Moles of vanadium=
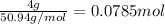
Total number of atoms of vanadium
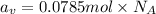
Total number of atoms in an alloy =
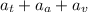
Atomic percentage:
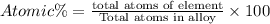
Atomic percentage of titanium:
:
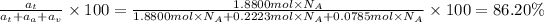
Atomic percentage of Aluminium:
:
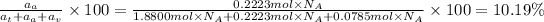
Atomic percentage of vanadium
: