Answer:
The pH of the solution is 12.31 .
Step-by-step explanation:
Initial molarity of barium hydroxide =

Initial volume of barium hydroxide =
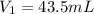
Final molarity of barium hydroxide =

Final volume of barium hydroxide =
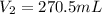

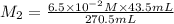
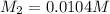
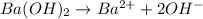
1 mol of barium gives 2 mol of hydroxide ions.
Then 0.0104 M of barium hydroxide will give:
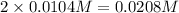 of hydroxide ions
of hydroxide ions
![[OH^-]=0.0208 M](https://img.qammunity.org/2020/formulas/chemistry/college/8vebvqn7ipja0ht40ibs3yzui65ngdxhsr.png)
![pH=14-pOH=14-(-\log[OH^-])](https://img.qammunity.org/2020/formulas/chemistry/college/xgd3rhhqjp6ncv9aiwfie2vo7tc9orgx0x.png)
![pH=14-(-\log[0.0208 M])=12.31](https://img.qammunity.org/2020/formulas/chemistry/college/76khw48m0rw0j6ppxob2bekw9zh19isgo9.png)
The pH of the solution is 12.31 .