Answer:
The pH of the final solution is 0.16 .
Step-by-step explanation:
The pH of the solution is defined as negative logarithm of hydrogen ion concentration in a solution.
![pH=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vz65x0ueuj8r8ibqa81zvsbzb2yaetlce4.png)
Concentration of HCl = 0.16 M
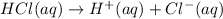
HCl is a string acid .1 molar of HCl gives 1 molar of of hydrogen ions.
![[H^+]=0.16 M](https://img.qammunity.org/2020/formulas/chemistry/college/8pqr50ittwh6zmk16mf6x8rprkf8bpomhw.png)
Concentration of
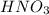 = 0.52 M
= 0.52 M
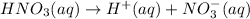
Nitric is a string acid .1 molar of nitric acid gives 1 molar of of hydrogen ions.
![[H^+]'=0.52 M](https://img.qammunity.org/2020/formulas/chemistry/college/w0548urbqjxpca1qp8ezbe3ajfl69g7xgm.png)
Total hydrogen ion concentration:
![[H^+]''=[H^+]+[H^+]'](https://img.qammunity.org/2020/formulas/chemistry/college/hep0bryka7y9drjutvkfm473m74pfxjgxw.png)
=0.16 M+0.52 M=0.68 M
The pH of the solution:
![\pH=-\log[H^+]''=-\log[0.68 M]](https://img.qammunity.org/2020/formulas/chemistry/college/l1hy99ajqj28j89927nt9flamucx6wqfz1.png)
pH = 0.16
The pH of the final solution is 0.16 .