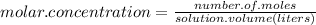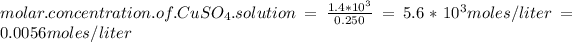Answer:
Molar concentration of
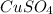 solution = 0.0056 moles / liter
solution = 0.0056 moles / liter
Step-by-step explanation:
Looking at the chemical reaction we realize that the precipitate, formed by adding the iron powder, is cooper.
Then for finding the number of moles of precipitated copper:
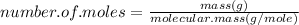
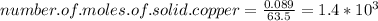
From the chemical reaction we deduce that
 moles of Cu equals to
moles of Cu equals to
 moles of
moles of
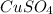 in the initial solution.
in the initial solution.
So molar concentration is defined as: