Answer:
0.2886 atm is the osmotic pressure of a solution.
Step-by-step explanation:
Osmotic pressure of solution =
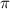
Concentration of the solution = c
Mass of the ammonium sulfate = 1.502 g
Moles of ammonium sulfate =
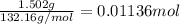
Volume of the solution = 1 L
Concentration of the solution:
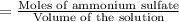
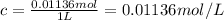
Temperature of the solution ,T= 36.54°C = 309.69 K
R = universal gas constant = 0.08206 L atm/mol K
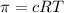
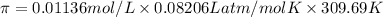
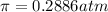
0.2886 atm is the osmotic pressure of a solution.