Answer:
The new rms speed is 2 times the original rms speed.
Step-by-step explanation:
The root‑mean‑square speed, rms, is related to temperature, , by the formula
 = √3 / ℳ
= √3 / ℳ
For a given gas,
 ∝ √
∝ √
or
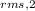 /
/
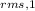 = √
= √
 /
/

In this case, is quadrupled.
√4 = 2
The new rms speed is 2 times the original rms speed.