Answer: 161.8 torr
Step-by-step explanation:
According to Raoult's law, the vapor pressure of a component at a given temperature is equal to the mole fraction of that component multiplied by the vapor pressure of that component in the pure state.
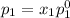 and
and
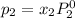
where, x = mole fraction
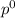 = pressure in the pure state
= pressure in the pure state
According to Dalton's law, the total pressure is the sum of individual pressures.
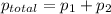
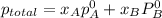
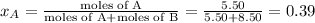 ,
,
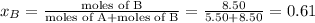 ,
,
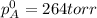
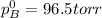
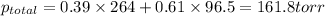
The total vapor pressure above the solution is 161.8 torr.