Answer:
The ratio of the temperature of helium to that of hydrogen gas is 2:1.
Step-by-step explanation:
Atomic mass of hydrogen = M
Temperature of hydrogen gas =T
Pressure of the hydrogen gas = P
Mass of the hydrogen gas = m
Moles of the hydrogen gas =
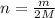
Volume of the hydrogen gas = V
Using an ideal gas equation:
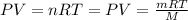 ...(1)
...(1)
Temperature of helium gas =T'
Pressure of the helium gas = P'= P
Mass of the helium gas = m' =m
Moles of the helium gas =
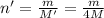
Volume of the helium gas = V' = V
Using an ideal gas equation:
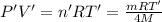 ...(2)
...(2)
Divide (2) by (1)
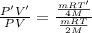
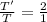
The ratio of the temperature of helium to that of hydrogen gas is 2:1.