Answer:
Dimer of two peptide chains with 1 mole of molybdenum metal each.
Step-by-step explanation:
Percentage of molybdenum in protein = 0.08%
Molecular mass of nitrate reductase = 240,000 g
Mass of molybdenum = x
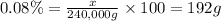
Moles of molybdenum =
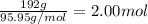
Each peptide chain of nitrate reductase contain 1 mole of molybdenum.
This means that nitrate reductase is composed of to two peptide chains. And in each peptide there is a single mole of molybdenum metal.