Step-by-step explanation:
When a non-volatile solute is added in a solvent then decrease in its freezing point is known as freezing point depression.
Mathematically,
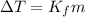
where
 = change in freezing point
= change in freezing point
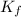 = freezing point depression constant
= freezing point depression constant
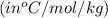
m = molality
First, calculate the number of moles as follows.
No. of moles =
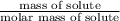
=
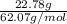
= 0.367 mol
Now, it is given that mass of solvent is 87.95 g. As there are 1000 grams in 1 kg.
So,
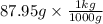 = 0.08795 kg
= 0.08795 kg
Hence, molality of the given solution is as follows.
Molality =
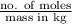
=
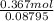
= 4.172 mol/kg
Therefore, depression in freezing point will be as follows.
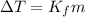
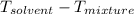 =
=
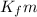
Since, freezing point of pure water is
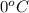 . Now, putting the given values as follows.
. Now, putting the given values as follows.
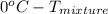 =
=
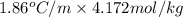
=
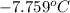
Thus, we can conclude that the freezing point of water in the given mixture is
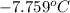 .
.