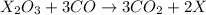Answer:The molecular formula of the oxide of metal be
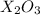 . The balanced equation for the reaction is given by:
. The balanced equation for the reaction is given by:
Step-by-step explanation:
Let the molecular formula of the oxide of metal be
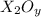
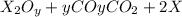
Mass of metal product = 1.68 g
Moles of metal X =
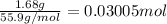
1 mol of metal oxide produces 2 moles of metal X.
Then 0.03005 moles of metal X will be produced by:
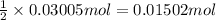 of metal oxide
of metal oxide
Mass of 0.01502 mol of metal oxide = 2.40 g (given)
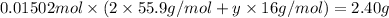
y = 2.999 ≈ 3
The molecular formula of the oxide of metal be
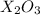 . The balanced equation for the reaction is given by:
. The balanced equation for the reaction is given by: