Answer: A group 1 alkali metal bonded to fluoride, such as LiF.
Step-by-step explanation:
Electronegativity is defined as the property of an element to attract a shared pair of electron towards itself. The size of an atom increases as we move down the group because a new shell is added and electron gets added up.
1. A strong acid made of hydrogen and a halogen, such as HCl : A polar covalent bond is defined as the bond which is formed when there is a difference of electronegativities between the atoms. Electronegativity difference = electronegativity of chlorine - electronegativity of hydrogen = 3-2.1= 0.9
2. A group 1 alkali metal bonded to fluoride, such as LiF: Ionic bond is formed when there is complete transfer of electron from a highly electropositive metal to a highly electronegative non metal.
Electronegativity difference = electronegativity of fluorine - electronegativity of lithium= 4-1= 3
3. Carbon bonded to a group 6A (16) nonmetal chalcogen, such as in CO: A polar covalent bond is defined as the bond which is formed when there is a difference of electronegativities between the atoms.
Electronegativity difference = electronegativity of oxygen - electronegativity of carbon= 3.5-2.5= 1.0
4. A diatomic gas, such as nitrogen
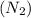 : Non-polar covalent bond is defined as the bond which is formed when there is no difference of electronegativities between the atoms.
: Non-polar covalent bond is defined as the bond which is formed when there is no difference of electronegativities between the atoms.
Electronegativity difference = 0
Thus the greatest electronegativity difference between the bonded atoms is in LiF.