Answer:
36.37% is the percent yield of the reaction.
Step-by-step explanation:
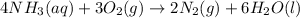
1)0.650 L nitrogen gas , at 295 K and 1.01 bar.
Let the moles of nitrogen gas be n.
Pressure of the gas ,P= 1.01 bar = 0.9967 atm (1 bar = 0.9869 atm)
Temperature of the gas = T = 295 K
Volume of the gas = V = 0.650 L
Using an ideal gas equation:

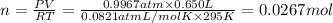
2) Moles of ammonia gas=
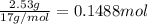
Moles of oxygen gas =
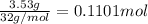
According to reaction ,3 mol of oxygen reacts with 4 mol of ammonia.
Then,0.1101 mol of oxygen will react with:
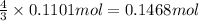 of ammonia.
of ammonia.
Hence, oxygen gas is in limiting amount and act as limiting reagent.
3) Theoretical yield of nitrogen gas :
According to reaction, 3 mol of oxygen gas gives 2 moles of nitrogen gas.
Then 0.1101 mol of oxygen will give:
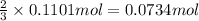 of nitrogen.
of nitrogen.
Theoretical yield of nitrogen gas = 0.0734 mol
Experimental yield of nitrogen as calculated in part (1) = 0.0267 mol
Percentage yield:
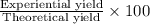
Percentage yield of the reaction:
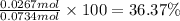
36.37% is the percent yield of the reaction.