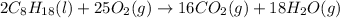Answer : The balanced chemical reaction will be,
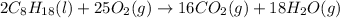
Explanation :
The process is a combustion reaction.
Combustion reaction : It is a type of reaction in which a hydrocarbon react with the oxygen gas to give carbon dioxide gas and water vapor as a product.
As per question, when liquid octane react with oxygen gas to give carbon dioxide gas and water vapor as a product.
Thus, the balanced chemical reaction will be :