Answer:
The formula of the compound will be:
![[Ru(Cl)_1(H_2O)_5]Cl_2](https://img.qammunity.org/2020/formulas/chemistry/high-school/x3wo61fja7kbzal7jwcy5z6102qkwqr6ya.png) .
.
Step-by-step explanation:
Coordination sphere is an array of ions or ligands around central atom or or an ion. Ions and ligands linked to central atom forms primary coordination sphere and ions or molecules linked to primary coordination sphere forms secondary coordination sphere.
Given: If one mole of
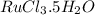 reacts with
reacts with
 to produce two moles of AgCl precipitate.
to produce two moles of AgCl precipitate.
This means that in primary coordination sphere there is a 1 chloride ion and 5 water molecules. And remaining 2 chloride ions present in secondary sphere which are available to react with silver ions of silver nitrate solution.
This why on reaction with silver nitrate 2 molecules or silver chloride are formed.
![[Ru(Cl)_1(H_2O)_5]Cl_2+2AgNO_3(aq)\rightarrow 2 AgCl(s)+[Ru(Cl)_1(H_2O)_5](NO_3)_2(aq)](https://img.qammunity.org/2020/formulas/chemistry/high-school/1lbbr62lcxeakx19q67umyxaofyjhrzujw.png)
The formula of the compound will be:
![[Ru(Cl)_1(H_2O)_5]Cl_2](https://img.qammunity.org/2020/formulas/chemistry/high-school/x3wo61fja7kbzal7jwcy5z6102qkwqr6ya.png) .
.