Answer: Option (C) is the correct answer.
Step-by-step explanation:
An ionic solid is defined as the solid in which atoms are chemically combined together due to transfer of electrons.
As all ionic substances are soluble in water. So, when an ionic solid is dissolved in water then it will dissociate into ions.
As a result, an equilibrium will be maintained between the solid and its ions into the solution.
For example,
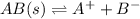
![K_(sp) = ([A^(+)])/([B^(+)])](https://img.qammunity.org/2020/formulas/chemistry/middle-school/1jrc8o3yd2tr82o32wey4suyy3o5r12hnc.png)
where,
 = solubility product
= solubility product
Thus, we can conclude that the information described by the solubility product constant is that there is equilibrium between the solid and its ions in solution.