Answer:
0.05 moles of sodium sulfate are required.
Step-by-step explanation:
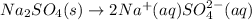
Concentration of sodium ions in 1 L = 0.10 M
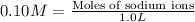
Moles of sodium ions = 0.10 mol
1 mole of sodium sulfate gives 2 moles of sodium ions.
Then 0.10 mol of sodium ions will be given by:
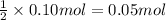
0.05 moles of sodium sulfate are required.