Answer:
![[Al^(3+)]](https://img.qammunity.org/2020/formulas/chemistry/college/7alerx4hp12zkqu9rvclpa6xcpb2kmxn98.png) = 1.834 M
= 1.834 M
![[F^-]](https://img.qammunity.org/2020/formulas/chemistry/college/noqezvgoz6etp25jnbbergzxym0k5ght9m.png) = 0.004 M
= 0.004 M
![[AlF_6^(3-)]](https://img.qammunity.org/2020/formulas/chemistry/college/6xt10z11snyhez0us0qu8spznlk3d7a4tg.png) = 0.166 M
= 0.166 M
Step-by-step explanation:
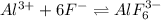
Initial concentration of
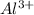 = 0.15 M
= 0.15 M
Initial concentration of
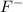 = 2.0 M
= 2.0 M
The given balanced equilibrium reaction is,
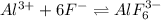
Initial conc. 2 M 0.15 M 0
At eqm. conc. (2-x) M (1-6x) M (x) M
The expression for equilibrium constant for this reaction will be,
![K_f=([<strong>AlF_6^(3-)</strong>])/([<strong>Al^(3+)</strong>][F^-]^6)](https://img.qammunity.org/2020/formulas/chemistry/college/9n7tzxqnba3rxfgywmoogefedns47k6g4p.png)
Now put all the given values in this expression, we get :
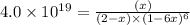
By solving the term 'x', we get :
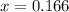
![[Al^(3+)]](https://img.qammunity.org/2020/formulas/chemistry/college/7alerx4hp12zkqu9rvclpa6xcpb2kmxn98.png) = (2-x) = 2-0.166 = 1.834 M
= (2-x) = 2-0.166 = 1.834 M
![[F^-]](https://img.qammunity.org/2020/formulas/chemistry/college/noqezvgoz6etp25jnbbergzxym0k5ght9m.png) = (1-6x) = 1-6(0.1660)= 0.004 M
= (1-6x) = 1-6(0.1660)= 0.004 M
![[AlF_6^(3-)]](https://img.qammunity.org/2020/formulas/chemistry/college/6xt10z11snyhez0us0qu8spznlk3d7a4tg.png) = x = 0.166 M
= x = 0.166 M