Answer : The correct option is, (A)
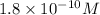 , basic.
, basic.
Explanation : Given,
Concentration of
 ion =
ion =
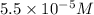
First we have to calculate the pOH.
![pOH=-\log [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/h1t4ubcsdqvqg0xpalkkvnwrun04y9pzd8.png)
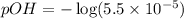
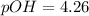
Now we have to calculate the pH.
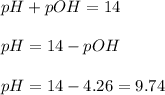
Now we have to calculate the
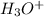 concentration.
concentration.
![pH=-\log [H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/xj6fwrpeduepfcp6k4cv1s827uf5c0pbp1.png)
![9.74=-\log [H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/college/h34cun2ex8r52l6apq8owuv8snvkbupiqw.png)
![[H_3O^+]=1.8* 10^(-10)M](https://img.qammunity.org/2020/formulas/chemistry/college/u0bh37ayrha590l0ervctidz91rwgzvrwd.png)
As we know that, when the pH value is less than 7 then the solution acidic in nature and when the pH value is more than 7 then the solution basic in nature.
From the pH value, 9.74 we conclude that the solution is basic in nature because the value of pH is greater than 7.
Therefore, the
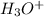 concentration is,
concentration is,
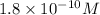 , basic.
, basic.