Answer:
173.83 mmHg is the vapor pressure of a ethylene glycol solution.
Step-by-step explanation:
Vapor pressure of water at 65 °C=
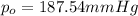
Vapor pressure of the solution at 65 °C=
The relative lowering of vapor pressure of solution in which non volatile solute is dissolved is equal to mole fraction of solute in the solution.
Mass of ethylene glycol = 22.37 g
Mass of water in a solution = 82.21 g
Moles of water=
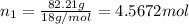
Moles of ethylene glycol=
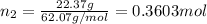
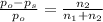
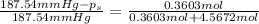
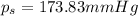
173.83 mmHg is the vapor pressure of a ethylene glycol solution.