Answer:
78.87 liters of bromine gas at 300 °C and 735 Torr are formed.
Step-by-step explanation:
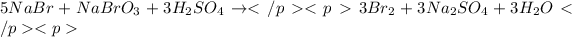
Moles of sodium bromide =
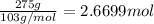
Moles of sodium bromate =
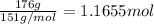
According to reaction , 1 mol of sodium bromate reacts with 5 moles of sodium bromide. Then 1.1655 mol of sodium bromate will react with:
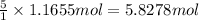 of sodium bromide.
of sodium bromide.
This means that sodium bromide is in limiting amount the amount of bromine gas depends upon sodium bromide.
According to reaction 5 moles of sodium bromide gives 3 moles of bromine gas.
Then 2.6699 moles of sodium bromide will give:
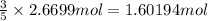 of bromine gas
of bromine gas
Volume occupied by bromine gas at 300 °C and 735 Torr.
Pressure of the gas = P =735 Torr = 0.9555 atm
Temperature of the gas = T = 300°C = 573 K
n = 1.60194 mol

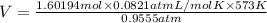
V = 78.87 L
78.87 liters of bromine gas at 300 °C and 735 Torr are formed.