Answer: The correct answer is it converts carbon monoxide faster.
Step-by-step explanation:
Catalyst is defined as the substance which increases the rate of the reaction without getting actually participated in the reaction. It can be easily regenerated at the end of the reaction.
For the given chemical reaction:
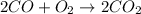
Addition of catalyst will lead us to the production of carbon dioxide faster or it may convert carbon monoxide faster into the products.
Hence, the correct answer is it converts carbon monoxide faster.