Answer:
Step-by-step explanation:
Given parameters:
pH = 3.50
Unknown:
concentration of [H₃0⁺] = ?
concentration of [OH⁻] = ?
Solution:
In order to find the unknown, we use some simple expressions which best explains the pH scale and the equilibrium systems of aqueous solutions.
pH = -log₁₀[H₃O⁺]
[H₃O⁺] = inverse log₁₀ (-pH) =
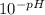 =
=
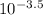
[H₃O⁺] = 3.2 x 10⁻⁴moldm⁻³
For the [OH⁻]:
we use : pOH = -log₁₀ [OH⁻]
Recall: pOH + pH = 14
pOH = 14 - pH = 14 - 3.5 = 10.5
Now we plug the value of pOH into pOH = -log₁₀ [OH⁻]
[OH⁻] =
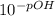
[OH⁻] =
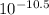 = 3.2 x 10⁻¹¹moldm⁻³
= 3.2 x 10⁻¹¹moldm⁻³
The solution is acidic as the concentration of H₃0⁺ is more than that of the OH⁻ ions.