Answer:
Number of electrons,
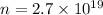
Step-by-step explanation:
It is given that,
Charge, q = 4.33 C
We need to find the number of electrons that make 4.33 C of charge. According to quantization of charge as :
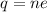
n = number of electrons
e = electron's charge
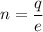
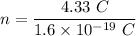
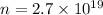
So, the number of electrons are
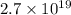 Hence, this is the required solution.
Hence, this is the required solution.